In April 2018
Digital Diagnostics Achieves a Historic Milestone
Digital Diagnostics became the first company to ever receive FDA clearance for an AI diagnostic platform that makes a diagnosis without physician input at the point-of-care.This achievement did not happen overnight – it was the culmination of decades of research by founder Dr. Michael Abramoff on automated image analysis, combined with decades of research on how clinicians diagnose disease.
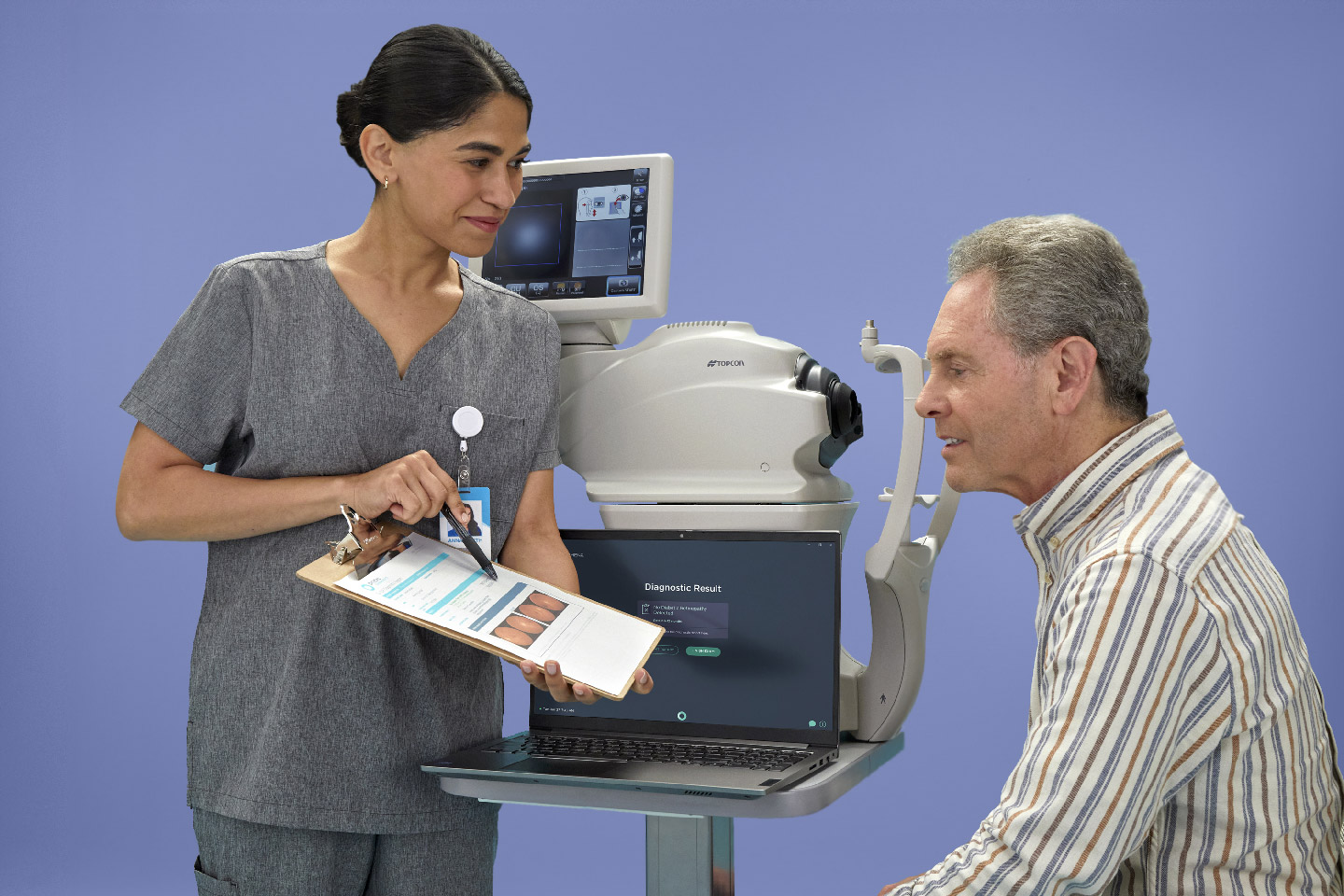
LumineticsCore (formerly IDx-DR) With Tablet Touch Screen

“Working With – Not Against – the Healthcare System”
Digital Diagnostics’ first FDA-cleared product is the AI diagnostic platform Dr. Abramoff had envisioned years earlier. The platform, called LumineticsCore™ (formerly IDx-DR), detects diabetic retinopathy (including macular edema) at the point-of-care. After completing a rigorous prospective, preregistered clinical trial at primary care sites across the country, LumineticsCore became the first FDA-cleared AI diagnostic system to make a diagnosis without physician input at the point-of-care. Since then, Digital Diagnostics has diligently worked from within the healthcare system to establish automated diagnosis as the new standard of care.
Advancing Automated Diagnosis Standards
From real-world clinic launch & EMR integration, to the creation of the first ever autonomous AI CPT® category 1 code for billing and payment, to inclusion in the American Diabetes Association (ADA)’s 2020 Standards of Care in Diabetes, Digital Diagnostics has proven that intelligent diagnostic platforms can be deployed safely and responsibly to improve patient outcomes and increase healthcare productivity.
Digital Diagnostics is in use at over 20 health systems and our customers have tested thousands of patients and identified hundreds of patients with disease who were previously undiagnosed.
Tomorrow, Digital Diagnostics is launching additional AI platforms for the detection of a wide range of disease states beyond the eye.